We offer strategic and efficient solutions for developing and managing biopharma drug development programs that effectively align with corporate objectives and goals. Our services are comprehensive, blending high-quality project management expertise with exceptional scientific knowledge.

20+ Years of Experience Across All Aspects of Biopharma Drug Development
- Program and Alliance Management
- IND enabling, Phase 1 to Phase 4 Project Management
- Clinical Strategic Planning
- Clinical Development Plan
- Target Product Profile
- Preclinical and CMC Management
- Long Range Planning
- Medical and Regulatory Writing (Protocol, IB, IND, NDA and BLA)
- CRO and CDMO selection and oversight

About Us
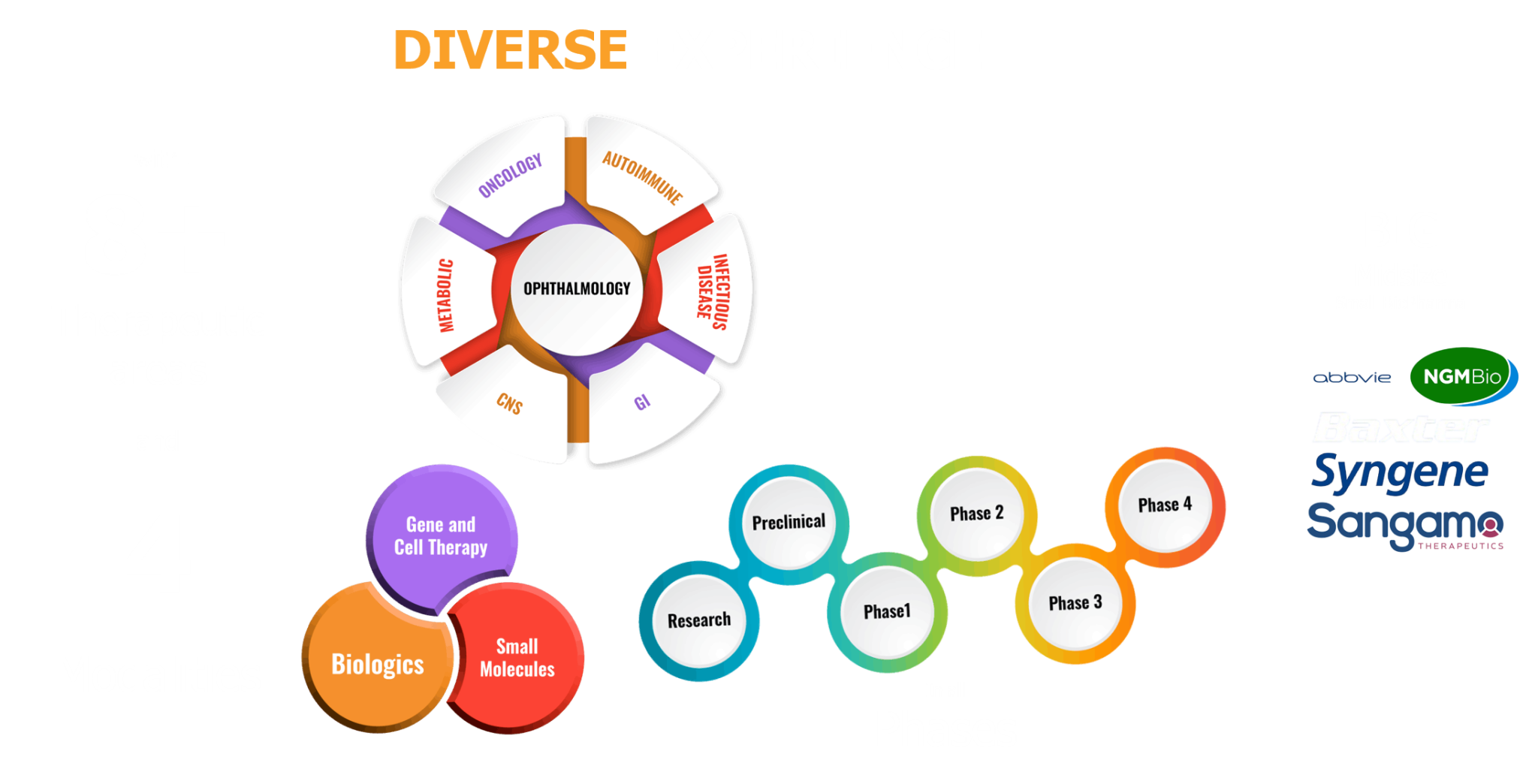
The SS BioPharma consulting provides strategic and operational support to the Biotech and Pharmaceutical industries in pursuit of their clinical development programs. The SS BioPharma consulting brings 20+ years of experience across all aspects of biopharma drug development in over eight therapeutic areas including oncology/Immuno-oncology, ophthalmology, infectious, metabolic, autoimmune, CNS, and gastrointestinal diseases using therapeutic modalities such as small and large molecules, gene and cell therapy.
We specialize in accelerating drug development from lead optimization through approval, with a core focus on preclinical, CMC, and clinical development project and program management.
Our Services


Project, Program, & Portfolio Management
Work closely with cross-functional project teams to develop strategies, plans, timelines, and budgets while identifying and mitigating risks to align with corporate goals.
Read More

Corporate Alliance Management & Business Development
Collaborate with project team leads and external biopharma partners to define alliance goals, establish core teams, facilitate meetings,
Read More

Vendor selection & oversight
Help identify and select domestic and global CRO/CDMO selection; provide tech transfer and study oversight including in vivo efficacy, GLP Tox, analytical assay method development and validation, process development,
Read More

Establish PMO
Develop Project Management Office (PMO) processes and systems to ensure a unified and efficient project portfolio. Integrate best practices, tools, and governance structures to streamline project management operations.
Read More

Technical support
Help with translational research and preclinical study design, bioassay development, and biomarkers identification. Develop Clinical Development Plan, and Target Product Profile (TPP).
Read More

Medical and Regulatory Writing, Biostatistics and Data Management
In collaboration with partners, prepare INDs, protocols, Investigator Brochures. and NDAs/BLA, and CSR, and provide biostatistics
Read MoreRecommendations
Dhananjay Patankar
Biopharmaceutical IndustrySyed worked with me in supporting a very strategic and critical relationship between Syngene and Baxter for pharmaceutical development. Syed was proactive in ensuring the relationship stayed smooth, and any risks were anticipated and mitigated before they could adversely impact the deliverables. The role demanded cross-functional and cross-geography collaboration. Syed was able to seamlessly engage with scientists in India and the US, applying his domain knowledge of pharmaceutical development, methodical approach in project management, his maturity of understanding, along with his interpersonal abilities. The team's success would not have been possible without Syed's contribution and management. Personally, Syed is very easy to get along and interact with while being very professional in his work. I recommend Syed for any professional endeavour he seeks.
Joe Yee
Director Business Operations Program Lead Gilead SciencesDuring his time with Sangamo Therapeutics, Syed reported directly to me. Syed as a hard worker, voracious learner and consummate professional, with a strong foundation of organization, planning, communication and project management skills to pair with his scientific and industry knowledge and experience. He keeps his teams organized, informed and focused on execution and escalation only when necessary. He does a good job of understanding project team needs and aligning them with program and corporate objectives and constraints. I wish Syed well, and recommend him for any professional endeavor that matches his strong qualifications.
David Kwon MBA, MD, PhD
Life Science & Healthcare Executive -Medical Affairs, Drug Development & Digital TransformationI had the pleasure of working with Syed at NGM Biopharma and recommend him with the highest regard. Syed brings many critical skills that help lead teams to success. Immediately, I was impressed with Syed's professionalism and the confidence with which he easily manages both project level meetings as well as program summary meetings with the executive leadership team. His command of the scientific mechanisms behind novel therapies, his experience guiding development programs efficiently, and his deep understanding of how all different stakeholder groups integrate towards the goal of regulatory approval all speak to the value Syed brought to the NGM Bio organization. Syed is proactive, organized, and detail oriented. At the same time, from his industry expertise and natural wisdom, he understands the greater context and can uncover new ways of achieving goals. Syed's many contributions to trial decision making, projecting program needs, and gathering appropriate resources and internal support all were vital to success. I remember enjoying many discussions about the biotech industry and learning from his specific scientific expertise in immuno-oncology. His thoughtful and confident leadership complement well the knowledge and skills he brings to our industry. He is a wonderful colleague and friend, and I look forward to how he will advance exciting programs and companies to great success.
Mike Sherrill
Executive Director, Project ManagementI had the opportunity to work with Syed in the Project Management group at NGM Bio. Syed managed multiple projects across therapeutic areas and in various stages of development. Syed was a technically skilled project manager and exhibited the interpersonal skills needed to be successful. He was able to work effectively with cross functional teams and helped to understand the project teams' needs and supported the teams to meet the project goals and deliverables. Syed is organized, detailed-oriented and has a strong understanding of the drug development process. Syed is willing to accept new challenges and embrace new opportunities to grow and develop as a drug development professional.
James O'Hare
PMP Biotech Project Management ProfessionalI worked with Syed at NGM Bio and recommend him for any drug development project management positions. His team-building skills and project management knowledge are impressive. Syed is proactive, organized, and detail-oriented and has a strong knowledge of the biopharmaceutical development process. He works to understand the project team's needs and goals to for inclusion in the development plan. He is professional with excellent communication and organization skills and interacts well with the whole team. The team's success would not have been possible without his contribution and management.
Arun T Saha
Investor | Board Member | Strategic Advisor | Digital Transformations | Innovation | Global Strategy |During my professional association with Syed, I was impressed with his scientific and project management skills. He was instrumental in building high performing cross functional teams and lending his scientific knowledge of the bio-pharmaceutical development process to ensure consistent program success. I valued his proactive communication, and detail-oriented approach to Project Management. Syed was also willing and able to accept new challenges and learn emerging technologies. I recommend Syed for project management as well as technical development roles.
News & Articles
Connecting Your Business To The Technology Resources You Need
Delivering Excellence Worldwide








